Abstract
Aim: Hepatitis C virus (HCV) is a potentially serious bloodborne virus, which persists in the majority of those infected. Long-term sequelae include liver cirrhosis, liver cancer and premature death. Early identification of newly acquired infection is important for protection of public health. Routine surveillance based on laboratory notification of HCV infection is not sufficient to differentiate between newly acquired and chronic infections. Enhanced surveillance systems have been trialled globally in a number of settings. This pilot program aimed to increase identification of newly acquired HCV cases in southeastern Sydney residents and to ascertain the likely mode of transmission.
Methods: All HCV notifications in southeastern Sydney residents with specimen dates from 1 July to 31 December 2012 were included in a pilot program. Demographic data, Australian Indigenous identification and previous laboratory results were collected from electronic medical records, where available. Enhanced surveillance forms were sent to referring doctors to seek information about clinical symptoms and previous hepatitis C pathology. Data were collated to assess, according to Australian national case definitions, whether cases were newly acquired or not, or were unable to be determined on the available information.
Results: There were 104 notifications of HCV infection during the surveillance period. Forms were sent to 100 requesting doctors, with 72 forms returned. Six newly acquired cases were identified, a rate of 8%, compared with 1–3% classified by routine surveillance. Twenty cases (28%) were not newly acquired and the status of 46 (64%) was unable to be determined. Of the six newly acquired cases, sexual transmission was deemed to be the likely route of exposure for four cases, and injecting drug use for the remaining two.
Conclusions: Enhanced surveillance increased the rate of identification of newly acquired infections. However, the process was labour-intensive and the status of most cases was unable to be determined. Since identification of newly acquired cases has an important public health benefit in understanding factors in disease transmission, other approaches should be examined.
Full text
Background
Hepatitis C affects around 300 000 people in Australia.1 Chronic hepatitis C infection, although often asymptomatic, has the potential to lead to liver cirrhosis, liver cancer and premature death. Most people who acquire hepatitis C virus (HCV) infection become chronically infected. Spontaneous clearance occurs in approximately 25% of those infected, but protective immunity is not conferred in this group and reinfection is possible.2 In 2011, more than 10 000 people were newly diagnosed with HCV infection in Australia; at least 400 had acquired the infection in the previous two years.1 At least half of the 300 000 people now infected are thought to have early liver disease, with 6300 estimated to have developed cirrhosis as a result of the infection.1 HCV infection is now the leading reason for liver transplant in Australia3, and has been estimated to cost $252 million annually, with a five-year projected cost of $1.5 billion.4
Injecting drug use has long been identified as the main mode of HCV transmission in Australia. In 2006, an estimated 82.3% of people with markers for HCV infection had been exposed through injecting drug use. A further 10.9% arrived in Australia already infected from countries with high HCV prevalence.5 Exposure routes also include blood transfusions and blood products (before 1990 in Australia), nonsterile tattooing or body piercing, and vertical transmission.6 Data suggest that, in Australia, only 2% of HCV infection is sexually acquired.6 However, recent reports suggest that sexual transmission may play a greater role, particularly in men who have sex with men (MSM) and who are co-infected with human immunodeficiency virus (HIV); this is an emerging focus of inquiry.7–10
Effective surveillance to identify newly acquired cases provides the opportunity to identify changes in transmission rates, identify infection patterns, detect outbreaks and develop disease burden projections to assist targeted prevention and control.
Hepatitis C surveillance in New South Wales
HCV infection is a notifiable condition in all Australian jurisdictions. In New South Wales (NSW), pathology laboratories are required to notify markers for HCV infection. Medical practitioners and hospitals are required to notify acute viral hepatitis on clinical suspicion. Data are collated in the NSW Notifiable Conditions Information Management System (NCIMS), a web-based database used by Health Protection NSW and public health units to manage notifiable disease data. Cases are classified in NCIMS as ‘newly acquired’ or ‘unspecified’ according to available information (see Box 1 for case definitions). A newly acquired case is one for which there is reliable evidence that the infection was acquired in the 24 months before the first positive result. In 2011, 3326 cases were notified in NSW, of which 1.5% were classified as newly acquired.11 This is consistent with previous Australian studies identifying 1–3% of HCV notifications as newly acquired infections.12–14
Box 1. Case definition for newly acquired hepatitis C in Australia
A confirmed case requires either:
- Laboratory definitive evidence OR
- Laboratory suggestive evidence AND clinical evidence.
Laboratory definitive evidence
- Detection of anti-hepatitis C antibody from a person who has had a negative anti-hepatitis C antibody test recorded within the past 24 months OR
- Detection of hepatitis C virus by nucleic acid testing from a person who has had a negative anti-hepatitis C antibody test result within the past 24 months OR
- Detection of anti-hepatitis C antibody from a child aged 18 to 24 months OR
- Detection of hepatitis C virus by nucleic acid testing in a child aged 1 to 24 months.
Laboratory suggestive evidence
Detection of anti-hepatitis C antibody, or hepatitis C virus by nucleic acid testing.
Clinical evidence
Clinical hepatitis within the past 24 months (where other causes of acute hepatitis have been excluded) defined as:
- Jaundice OR
- Bilirubin in urine OR
- Alanine transaminase (ALT) seven times the upper limit of normal.
Source: Australian national notifiable diseases case definitions15
Routine surveillance for HCV infection through notification does not adequately lead to identification of newly acquired cases.16 Surveillance for newly acquired cases is complicated because the disease is often asymptomatic and there is no assay available to allow diagnosis of recent infection.16 To reliably classify cases as newly acquired or otherwise, it is necessary to assess clinical information and any relevant previous laboratory results. A recent global literature review16 indicated that creating surveillance systems to distinguish newly acquired HCV infections from those that are chronic is difficult and not well described in the literature. Only 15 countries had documented surveillance systems that were designed to differentiate newly acquired cases. Those systems included follow-up of all cases, follow-up of a random subset, and follow-up of a subset selected on factors such as age or presence of clinical or laboratory indicators. Although these systems increased ascertainment rates of newly acquired cases, they were not able to accurately estimate the true incidence due to lack of data on many cases.
Southeastern Sydney enhanced surveillance pilot project
The South Eastern Sydney Local Health District includes highly urban and industrial areas, as well as more suburban communities; its population of around 830 000 people is culturally and linguistically diverse. In 2011, there were 334 new HCV notifications in southeastern Sydney residents (10% of NSW notifications), of which 10 (3%) were confirmed to be newly acquired.11 In 2012, the South Eastern Sydney Public Health Unit conducted an enhanced surveillance pilot program. This was in part due to an acceptance that routine surveillance was not able to adequately capture newly acquired cases. It was also in recognition of considerable populations of people who inject drugs and MSM who were residing in the area.
Aim
The enhanced surveillance pilot project aimed to increase identification of newly acquired HCV cases in southeastern Sydney residents and ascertain the likely mode of transmission. A secondary aim of the project was to describe the characteristics of all confirmed HCV cases notified during the pilot period.
Methods
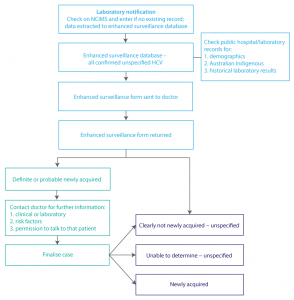
The period of the pilot was 1 July to 31 December 2012. The process is described in Figure 1. All laboratory notifications of a positive HCV result with a specimen date during this period were checked by surveillance officers and details entered into a stand-alone database. Electronic medical records were accessed to collect demographic data (country of birth, Indigenous identity and preferred language) and relevant previous laboratory results, where available.
A surveillance form with a covering letter was sent to each referring doctor. Information sought included demographic data, whether the patient had a clinical illness consistent with acute hepatitis in the previous 24 months, and whether the person had previous markers of hepatitis C infection. Returned forms were checked and cases confirmed to be residents in southeastern Sydney had their information entered in the database. Details of cases who resided elsewhere were passed on to the relevant health district.
Information recorded on the surveillance forms was considered, together with any available laboratory records, to determine whether a case was newly acquired or unspecified according to the case definitions (see Box 1). If there was laboratory evidence of previous HCV infection more than 24 months before the test date, the case was considered to not be newly acquired – an additional category used in the pilot but not available in NCIMS. If it was not possible to classify the infection, a follow-up phone call was made to the referring doctor to seek further clinical or laboratory data and, if necessary, ask permission to directly contact the case. Where newly acquired infection was suspected or confirmed, the doctor was asked for information about potential risk factors. HIV status was not requested, as the NSW Public Health Act 2010 currently prohibits disclosure under these circumstances without the patient’s consent. An amendment to public health legislation is under consideration that would allow provision of this information without gaining consent from the patient.
When all available data had been collected from surveillance forms, electronic records and follow-up phone calls, each case was classified and recorded in the database as either newly acquired, not newly acquired or unable to be determined.
Figure 1. Enhanced surveillance process (click to enlarge)
HCV = hepatitis C virus; NCIMS = Notifiable Conditions Information Management System
Results
Characteristics of hepatitis C notifications
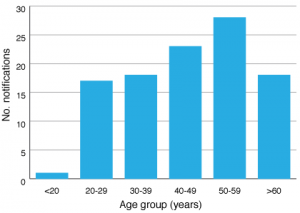
There were 104 notifications of HCV infection in southeastern Sydney residents during the pilot, an average of 17.3 per month (range: 12–25). The majority of cases were male (72%) and ages ranged from three years to 85 years. Notifications were most common in the 40–49 and 50–59 years age groups (Figure 2). Four cases were identified as Indigenous, 88 as non-Indigenous, and 12 were unable to have their Indigenous status confirmed.
Figure 2. Hepatitis C virus notifications in the surveillance period by age group (click to enlarge)
The most frequent referrers were general practitioners, followed by clinicians from public hospital clinics, sexual health clinics and emergency departments. The public hospital clinic doctors who notified cases were most commonly in gastroenterology but included a number of other disciplines. Doctors from private clinics, homeless persons’ clinics, HIV researchers and an Aboriginal Medical Service also provided a small number of notifications.
One hundred letters were sent to referring doctors. Letters were not sent for four cases as they were either considered inappropriate (in two cases, notifications were from a forensic pathologist) or unlikely to yield useful information (in two cases, notifications were from insurance companies). A total of 72 forms were returned, of which 61 (85%) were returned within two months. The demographic characteristics of patients notified with hepatitis C for whom no form was returned were broadly similar to those with a returned form (Table 1).
Table 1. Selected characteristics of cases with and without forms returned
| Surveillance form returned | ||
| Characteristic | Yes (n=72) | No (n=28) |
| Male | 68% | 82% |
| Average age | 45 years | 49 years |
| Australian Indigenous | 0.7% | 0.6% |
| Australian non-Indigenous | 54% | 57% |
The pilot project identified six newly acquired cases of HCV infection, one of which was also identified as a routine clinical notification of acute hepatitis. This represented 8% of the 72 cases with completed forms. Of the 66 remaining cases with returned forms, 20 (28%) were not newly acquired and 46 (64%) were unable to be determined.
The six newly acquired cases were confirmed on the basis of combined clinical and laboratory data. Three cases had documented negative HCV antibody results in the previous 24 months, and one of these also had HCV RNA detected at the time of diagnosis. Two other cases had both clinical symptoms consistent with acute hepatitis and documented previous negative HCV antibodies in the 24 months before their illness. The final case had a previous infection, with documentary evidence of successful treatment with virological clearance and subsequent infection with a different genotype.
Sexual transmission was identified as the likely mode of transmission for four cases. Two cases were acquired through injecting drug use, one of whom was an Indigenous person.
Discussion
Enhanced surveillance was shown to be effective in increasing identification of newly acquired cases. All but one of the cases identified through the pilot were not picked up by routine notification.
Although the pilot system increased identification of newly acquired cases, there remained a large number of cases that were unable to be classified due to lack of laboratory evidence. This is consistent with the international literature.16 The rate of 8% was an increase on published rates of 1–3% based on routine notification systems12–14, but evidence suggests this is still likely to be an underestimate.13 The large number of unclassified cases despite enhanced surveillance demonstrates the weaknesses of these systems.
In considering future surveillance responses, it is important to consider cost. Reports of similar systems internationally have suggested that this type of enhanced surveillance system is expensive and labour-intensive. The authors of a Victorian study estimated it would take 17.8 days per week (more than three full-time surveillance officers) to follow up all notifications in Victoria, while a 10% sample would take 2.9 days per week for a yield of 14 cases.13
Several potential approaches to improving identification rates of newly acquired cases have been described. Asking pathology laboratories to provide previous hepatitis C test results and subjecting them to periodic data linkage with the notification database, or providing previous results routinely at the time of a new notification have been suggested as less labour-intensive ways of identifying new infections.14,16 Another approach is to follow up only a sample of notifications. A targeted enhanced surveillance system in Victoria followed up two different types of sample, one random and the other specific. When a random 10% sample was followed up, 6% of cases were found to be newly acquired, 15% were not and the rest could not be classified, similar to the results in our pilot. A more targeted sample included all 16–19 year olds (based on evidence of injecting drug use as the main mode of transmission and the median age at initiation into injecting drug use) and those with markers of acute infection; in this sample, 36% were confirmed as newly acquired cases.17
The public health unit surveillance team was able to ascertain the likely mode of transmission for all newly acquired cases using enhanced surveillance. The incomplete data do not allow for population estimates, although the high number of cases reported to be due to sexual transmission is notable. It is possible that this is a reflection of the inner-city location of the health district, with several sexual health clinics serving a large client base of MSM; however, further study would be required to ascertain true rates and understand the dynamics of transmission.
Conclusion
Routine surveillance often fails to identify cases of new HCV infection or provide any information about current disease epidemiology that might inform public health interventions. Enhanced surveillance is able to increase identification of newly acquired HCV infections and provide important population-level information about risk factors and mechanisms of transmission, but is labour-intensive. Choosing the enhanced surveillance approach requires taking into account available resources, the context and the goals of surveillance.
Copyright:
© 2015 Bateman-Steel et al. This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International Licence, which allows others to redistribute, adapt and share this work non-commercially provided they attribute the work and any adapted version of it is distributed under the same Creative Commons licence terms.
References
- 1. The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia annual surveillance report 2012. Sydney: University of New South Wales; 2012 [cited 2015 Mar 17]. Available from: kirby.unsw.edu.au/surveillance/2012-annual-surveillance-report-hiv-viral-hepatitis-stis
- 2. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13(1):34–41. PubMed
- 3. Australia & New Zealand Liver Transplant Registry. ANZLT R 23rd Report. Brisbane: The Registry; 2011 [cited 2014 Jun 3]. Available from: www.anzltr.org/Reports/23rdReport.pdf
- 4. The Boston Consulting Group. The economic impact of hepatitis C in Australia. Canberra: The Boston Consulting Group; 2012 [cited 2014 Jun 3]. Sponsored by Janssen Australia. Available from: static.squarespace.com/static/50ff0804e4b007d5a9abe0a5/t/51ca2984e4b01fa56e27de4d/1372203396232/The%20Economic%20Impact%20of%20Hepatitis%20C%20in%20Australia_FINAL.pdf
- 5. Ministerial Advisory Committee on AIDS, Sexual Health and Hepatitis: Hepatitis C Sub-committee. Hepatitis C virus projections working group: estimates and projections of the hepatitis C virus epidemic in Australia 2006. Sydney: University of New South Wales; 2006 [cited 2014 Jun 3].
- 6. Dore GJ, Law M, MacDonald M, Kaldor J. Epidemiology of hepatitis C virus infection in Australia. J Clin Virol. 2003;26(2):171–84. PubMed
- 7. Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology. 2010;52(4):1497–1505. CrossRef | PubMed
- 8. van de Laar TJW, Matthews G, Prins M, Danta M. Acute hepatitis C in HIV–infected men who have sex with men: an emerging sexually transmitted infection. AIDS. 2010;24(12):1799–1812. CrossRef | PubMed
- 9. Mahony AA, Donnan EJ, Lester RA, Doyle JS, Knox J, Tracy SL, et al. Beyond injecting drug use: investigation of a Victorian cluster of hepatitis C among HIV– infected men who have sex with men. Med J Aust. 2013;198(4):210–14. CrossRef | PubMed
- 10. Matthews GV, Pham ST, Hellard M, Grebely J, Zhang L, Oon A, et al. Patterns and characteristics of hepatitis C transmission clusters among HIV–positive and HIV–negative individuals in the Australian trial in acute hepatitis C. Clin Infect Dis. 2011;52(6)803–11. CrossRef | PubMed
- 11. Health Protection NSW. Year in review: health protection in NSW, 2012. NSW Public Health Bull. 2013;24(3):105–18. CrossRef
- 12. Robotin M, Copland J, Tallis G, Coleman D, Giele C, Carter L, et al. Surveillance for newly acquired hepatitis C in Australia. J Gastroenterol Hepatol [Internet]. 2004;19:283–8. PubMed
- 13. Tobin S. Hepatitis C: enhancing routine surveillance in Victoria. Victorian Infectious Diseases Bulletin. 2001 [cited 2014 Jun 3];4(2):17–19.
- 14. Deacon RM, Wand H, Treloar C, Maher L. Improving surveillance for acute hepatitis C. Commun Dis Intell Q Rep. 2011 [cited 2014 Jun 3];35(1):16–20. Available from: www.health.gov.au/internet/main/publishing.nsf/Content/cda-cdi3501d.htm PubMed
- 15. The Department of Health. Australian national notifiable diseases case definitions – hepatitis C (unspecified). Canberra: Australian Government Department of Health; 2014 [cited 2014 Jun 3]. Available from: www.health.gov.au/internet/main/publishing.nsf/Content/cda-surveil-nndss-casedefs-cd_hepcun.htm
- 16. Sack-Davis R, Van Gemert C, Bergeri I, Stoove M, Hellard M. Identifying newly acquired cases of hepatitis C using surveillance: a literature review. Epidemiol Infect. 2012;140(11):1925–34. CrossRef | PubMed
- 17. Guy R, Devadason D, Lim M, Higgins N, Pedrana A, Gibson K, et al. Enhanced case detection for newly acquired hepatitis C infection: epidemiological findings and health service implications. Commun Dis Intell Q Rep. 2008 [cited 2014 Jun 3];32(2):250–6. Available from: www.health.gov.au/internet/main/publishing.nsf/Content/cda-cdi3202g.htm PubMed